The intricate structure of DNA is foundational to understanding the essence of life itself. At the heart of this double helix are the bonds that hold the strands together, ensuring stability and functionality. These bonds are not only vital in maintaining the physical structure of DNA but also play a crucial role in the processes of replication and transcription. Exploring the various types of bonds in DNA structure reveals the sophistication of molecular biology and underscores the importance of DNA in heredity and evolution.
In this article, we will delve into the types of bonds that comprise the DNA structure, such as hydrogen bonds and covalent bonds, and their significance in the overall stability and functionality of DNA. Understanding these bonds is essential for grasping how genetic information is stored, replicated, and expressed in living organisms. From the formation of the iconic double helix to the processes that ensure genetic continuity, the bonds in DNA structure are fundamental to all biological systems.
As we unfold the layers of DNA, we will address common questions surrounding its structure and function, providing a comprehensive overview of how these bonds interact to create the blueprint of life. Join us in this exploration of the molecular wonders that define our genetic code and the bonds in DNA structure that make it all possible.
What are the Types of Bonds in DNA Structure?
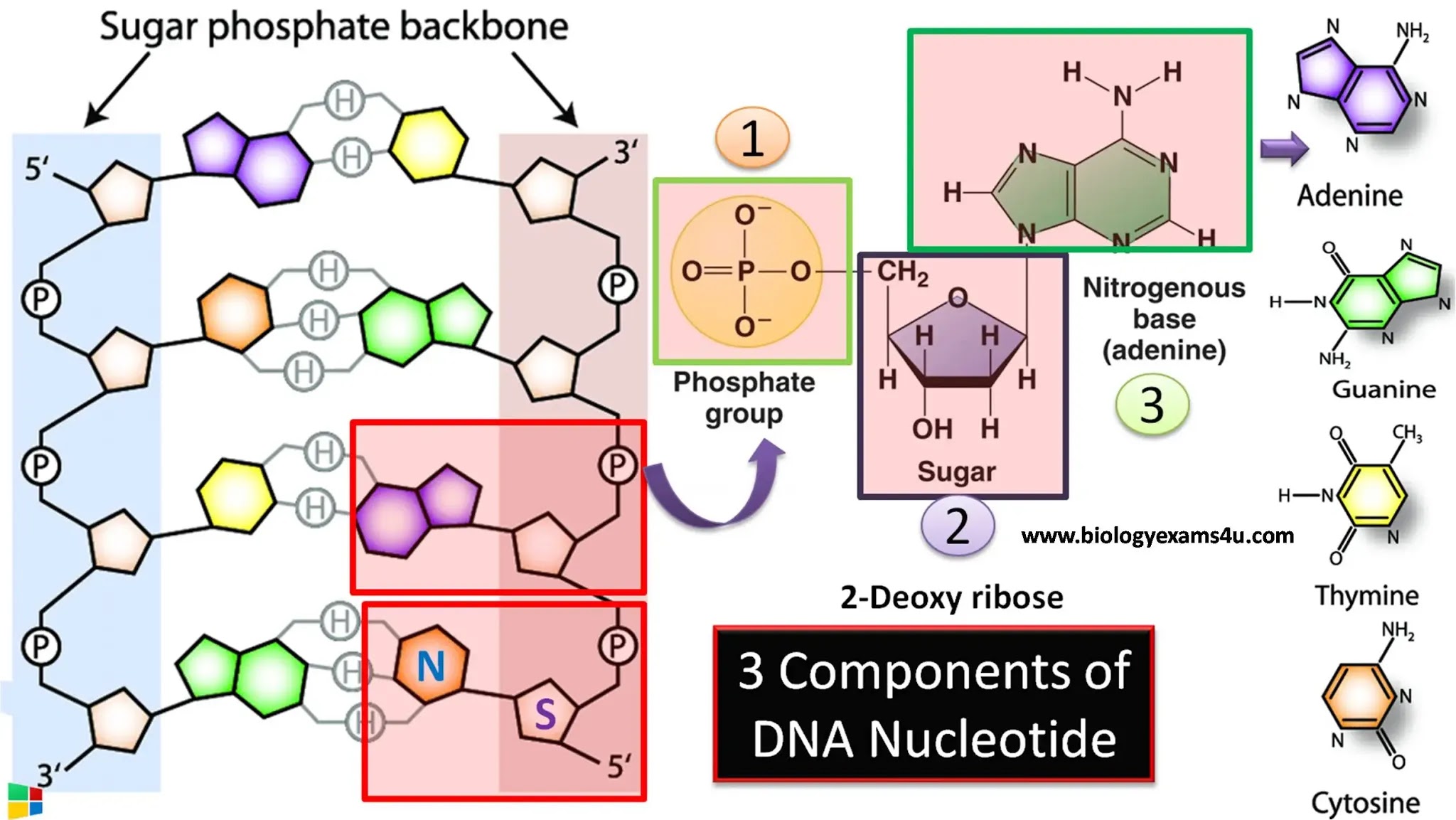
DNA contains two primary types of bonds that play critical roles in its structure:
- Covalent Bonds: These bonds link the sugar and phosphate groups of the DNA backbone, creating a strong and stable structure.
- Hydrogen Bonds: These weaker bonds form between the nitrogenous bases on opposite strands, allowing the double helix to maintain its shape while also enabling the strands to separate during replication.
Why are Covalent Bonds Important in DNA?
Covalent bonds are essential for maintaining the integrity of the DNA molecule. The strong link between the sugar and phosphate groups forms the backbone of DNA, ensuring that the structure is robust enough to withstand various cellular processes. This stability is crucial during DNA replication, where the integrity of the genetic code must be preserved. Without covalent bonds, the DNA would not be able to maintain its double helical shape, making it vulnerable to damage.
How Do Hydrogen Bonds Contribute to DNA Functionality?
Hydrogen bonds are pivotal in allowing the strands of DNA to separate and reconnect. They provide just the right amount of strength to maintain the double helix while still allowing the strands to unwind during replication and transcription. This property is essential for the accurate copying of genetic information. Additionally, the specificity of hydrogen bonding between complementary bases (adenine with thymine and guanine with cytosine) is crucial for the fidelity of genetic information transfer.
What Role Do Base Pairing and Hydrogen Bonds Play in DNA Structure?
The specific pairing of nitrogenous bases is a cornerstone of DNA structure. Hydrogen bonds facilitate this pairing, with adenine forming two hydrogen bonds with thymine and guanine forming three hydrogen bonds with cytosine. This complementary base pairing not only stabilizes the DNA structure but also ensures that genetic information can be accurately replicated and expressed. The precise arrangement of these base pairs contributes to the overall functionality of DNA, allowing for the encoding of complex genetic instructions.
How Do Bonds in DNA Structure Affect Genetic Variation?
The bonds in DNA structure, particularly the hydrogen bonds between base pairs, play a significant role in genetic variation. Mutations, which can occur during DNA replication, may involve changes in the sequence of bases. These changes can lead to alterations in the strength of hydrogen bonds, potentially impacting the stability of the DNA molecule. Understanding these variations and the underlying molecular mechanisms is crucial for fields such as genetics, evolution, and medicine.
Can the Bonds in DNA Structure Be Altered?
Indeed, the bonds in DNA structure can be altered through various means, including environmental factors, chemical exposure, and biological processes. For instance, certain chemicals can disrupt hydrogen bonding, leading to mutations. Moreover, enzymes involved in DNA repair can modify covalent bonds, ensuring the maintenance of genetic integrity. The ability of DNA to undergo changes while still maintaining its fundamental structure is a testament to its resilience and adaptability.
What is the Significance of Understanding Bonds in DNA Structure?
Understanding the bonds in DNA structure is crucial for several reasons:
- Advancements in Genetic Research: A deeper comprehension of DNA bonds aids in the exploration of genetic diseases and the development of targeted therapies.
- Biotechnology: Knowledge of DNA structure is essential for biotechnological applications, including gene editing techniques like CRISPR.
- Evolutionary Biology: Insights into DNA bonding mechanisms contribute to our understanding of evolutionary processes and genetic diversity.
How Do Bonds in DNA Structure Relate to Modern Medicine?
In modern medicine, the bonds in DNA structure are pivotal in various applications, including diagnostics, therapeutics, and personalized medicine. For instance, understanding the molecular basis of genetic diseases allows for the development of gene therapies that can correct specific mutations. Moreover, advances in DNA sequencing technology rely on the knowledge of DNA bonds to accurately read genetic information, leading to better disease management and treatment strategies.
What Future Research Directions Focus on Bonds in DNA Structure?
Future research will likely continue to explore the bonds in DNA structure, particularly in the context of synthetic biology and nanotechnology. Scientists are investigating ways to manipulate these bonds to create new forms of DNA that can perform specific functions or even self-assemble into complex structures. Additionally, ongoing studies into the effects of environmental factors on DNA bonding will enhance our understanding of genetics and its implications for health and disease.
In conclusion, the bonds in DNA structure are fundamental to the integrity and functionality of life itself. From the covalent bonds that form the backbone of the molecule to the hydrogen bonds that stabilize its double helix, these connections are essential for the accurate replication and expression of genetic information. As research continues to unravel the complexities of DNA, understanding these bonds will remain a cornerstone of molecular biology and genetics.
Exploring Bolly4u Org Bollywood 2024: Your Gateway To Hindi Cinema
Mastering The Art Of Bitcoin Mixing: A Comprehensive Guide
Exploring The Perks Of United Fully Refundable Tickets