Deoxyribonucleic acid, or DNA, is the molecular blueprint of life, encapsulating the genetic instructions necessary for the development and functioning of all living organisms. At the core of this intricate structure lies a fascinating array of chemical bonds that play critical roles in maintaining its stability and integrity. Understanding these bonds is essential for delving into the mysteries of genetics, evolution, and biotechnology. In this article, we will explore what types of bonds hold DNA together, shedding light on their significance and functions.
The DNA molecule is composed of two long strands that coil around each other to form a double helix. This unique structure is stabilized by various types of bonds, each contributing to the overall stability and functionality of the DNA molecule. The interplay of these bonds not only allows DNA to store and transmit genetic information but also enables it to undergo replication and repair. Therefore, understanding what types of bonds hold DNA together is not only a matter of scientific curiosity but also a foundational aspect of molecular biology.
As we delve deeper into the types of bonds that maintain the integrity of DNA, we'll uncover the mechanisms behind its stability and how these interactions impact biological processes. From the robust hydrogen bonds that connect base pairs to the covalent bonds that link the sugar-phosphate backbone, each bond serves a unique purpose. Join us as we explore the various bonds that hold DNA together, their characteristics, and their essential roles in the realm of life sciences.
What Are the Main Types of Bonds That Hold DNA Together?
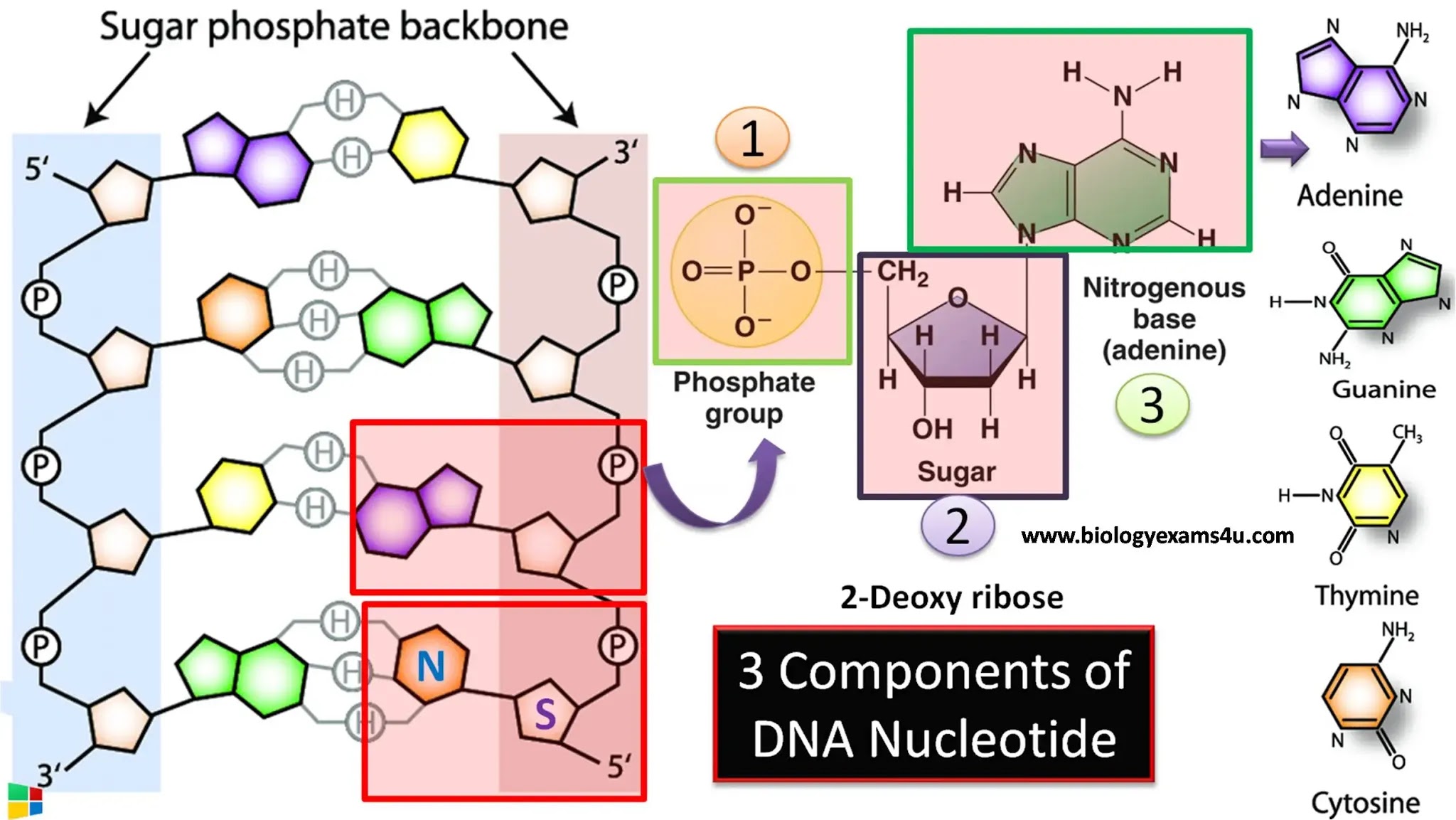
To understand what types of bonds hold DNA together, we must first examine the structural components of DNA. The basic building blocks of DNA are nucleotides, each consisting of a sugar molecule, a phosphate group, and a nitrogenous base. The bonds that hold these nucleotides together can be categorized into two main types: covalent bonds and non-covalent bonds.
1. What Are Covalent Bonds in DNA?
Covalent bonds are strong chemical bonds formed when atoms share electrons. In DNA, covalent bonds are crucial for linking the nucleotides together to form the DNA strands. Specifically, they exist between the phosphate group of one nucleotide and the sugar of the adjacent nucleotide, creating a sugar-phosphate backbone. This backbone is vital for maintaining the overall structure of DNA and ensuring its stability.
How Do Covalent Bonds Contribute to DNA Structure?
The sugar-phosphate backbone, held together by covalent bonds, provides a sturdy framework for the DNA molecule. The strength of these bonds means that the DNA strands can withstand significant environmental stress, which is essential for the preservation of genetic information. Thus, covalent bonds are fundamental in maintaining the integrity of DNA, allowing it to function as a stable repository of genetic data.
What Are Hydrogen Bonds in DNA?
In addition to covalent bonds, hydrogen bonds play a critical role in the structure of DNA. Hydrogen bonds are weaker than covalent bonds and occur when a hydrogen atom covalently bonded to one electronegative atom is attracted to another electronegative atom. In the context of DNA, hydrogen bonds form between complementary nitrogenous bases on opposite strands.
2. Why Are Hydrogen Bonds Important for DNA Stability?
The specific pairing of nitrogenous bases—adenine with thymine and guanine with cytosine—is facilitated by hydrogen bonds. Adenine and thymine form two hydrogen bonds, while guanine and cytosine form three. Despite their relative weakness compared to covalent bonds, these hydrogen bonds are crucial for stabilizing the double helix structure of DNA. They allow the strands to separate during replication and transcription while ensuring that the strands can re-anneal afterward.
How Do Hydrogen Bonds Affect DNA Replication?
The ability of DNA strands to separate due to the weaker hydrogen bonds is vital for the replication process. When DNA is replicated, the hydrogen bonds between the nitrogenous bases break, allowing the strands to unwind and serve as templates for the synthesis of new strands. This unique property of hydrogen bonds enables the accurate copying of genetic information, a cornerstone of heredity.
What Are the Roles of Ionic Bonds in DNA?
While most discussions about the bonds that hold DNA together focus on covalent and hydrogen bonds, ionic bonds also play a role in the stability of DNA structures, particularly in interaction with proteins and other molecules. Ionic bonds occur between positively charged and negatively charged ions, contributing to the overall stability of the DNA structure, especially in the context of chromatin packaging.
3. How Do Ionic Bonds Contribute to DNA-Chromatin Interactions?
The presence of positively charged histone proteins allows for ionic interactions with the negatively charged phosphate groups in the DNA backbone. These ionic bonds help package DNA into the compact structure of chromatin, which is crucial for fitting the long DNA molecules into the nucleus of cells. This interaction is essential for gene regulation and expression, as it influences the accessibility of DNA for transcription.
How Do Environmental Factors Affect DNA Bonds?
The integrity of the bonds that hold DNA together can be influenced by various environmental factors, including temperature, pH, and the presence of chemical agents. Understanding how these factors interact with DNA bonds is essential for fields such as genetics, molecular biology, and biotechnology.
4. What Environmental Factors Can Disrupt DNA Bonds?
Several environmental stresses can disrupt the bonds that hold DNA together, leading to potential mutations or structural damage. Some of these factors include:
- High temperatures that can lead to the denaturation of DNA.
- Extreme pH levels that can affect the stability of hydrogen bonds.
- Chemical agents, such as certain drugs or toxins, that can induce breaks in covalent bonds.
How Do Cells Repair DNA Damage?
Cells have evolved sophisticated mechanisms to repair DNA damage caused by environmental factors. These repair processes often involve recognizing broken covalent bonds and re-establishing the appropriate hydrogen bonds between bases. Understanding these repair mechanisms is essential for developing therapies for genetic disorders and cancer treatment.
Conclusion: The Importance of Bonds in DNA Structure
In summary, understanding what types of bonds hold DNA together is crucial for comprehending the molecular basis of life. Covalent bonds create a robust backbone that supports the structure of DNA, while hydrogen bonds provide the necessary flexibility for replication and transcription. Additionally, ionic bonds play a role in chromatin structures, further emphasizing the complexity of DNA. These bonds are not merely structural; they are fundamental to the processes of heredity, gene expression, and cellular function. As research continues to uncover the intricacies of these bonds, we gain deeper insights into the molecular mechanisms that underlie life itself.
Mastering Date Formats In Excel: A Comprehensive Guide
Discovering Seznam CZ: Najdu Tam
Exploring The Mystical Realm Of Dragon Ball Anime Saturn